Floor standing autoclaves for laboratory and production
Floor-standing units with 150 to 1,200 litre chamber volume
Our floor standing autoclaves are powerful, compact and economical for use in laboratories and pharmaceuticals. Due to the wide range of options they offer the right solution for every application.
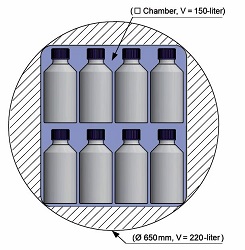
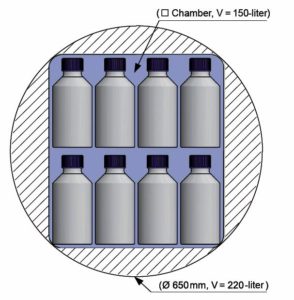
Autoclaves from 150 to 304 litres chamber volume
Compact units with square sterilization chamber and space-saving vertical door with fully automatic locking system. The rectangular chamber allows optimal use and a high loading capacity with small external dimensions.
| Type | HST 4x4x6 | HST 4x6x6 | HST 4x6x9 | HST 6x6x6 |
|---|---|---|---|---|
| Free usable space dimensions (mm) | (W)450 (H)500 (D)670 |
(W)450 (H)700 (D)670 |
(W)450 (H)700 (D)970 |
(W)650 (H)700 (D)670 |
| chamber volume (litres) | 150 | 210 | 305 | 304 |
| external dimensions in the single-door version (mm) |
(W)800 (H)1950 (D)1100 |
(W)800 (H)1950 (D)1100 |
(W)980 (H)1950 (D)1400 |
(W)980 (H)1950 (D)1100 |
Autoclaves from 440 to 849 litres chamber volume
They provide an excellent ratio between usable space and space requirements. Nevertheless, they can be fully equipped with customer-oriented system solutions to complete their sterilization tasks quickly, safely and reliably.
| Type | HST 6x6x9 | HST 6x6x12 | HST 6x6x16 | HST 6x6x18 |
|---|---|---|---|---|
| Free usable space dimensions (mm) | (W)650 (H)700 (D)970 |
(W)650 (H)700 (D)1270 |
(W)650 (H)700 (D)1670 |
(W)650 (H)700 (D)1870 |
| chamber volume (litres) | 440 | 577 | 758 | 849 |
| external dimensions in the single-door version (mm) |
(W)980 (H)1950 (D)1400 |
(W)980 (H)1950 (D)1700 |
(W)980 (H)1950 (D)2100 |
(W)980 (H)1950 (D)2300 |
Autoclaves from 370 to 1.030 litres chamber volume
| Typ | HST 8x6x6 | HST 8x6x9 | HST 8x6x12 | HST 8x6x14 | HST 8x6x16 | HST 8x6x18 |
|---|---|---|---|---|---|---|
| Free usable space dimensions (mm) | (W)650 (H)850 (D)670 |
(W)650 (H)850 (D)970 |
(W)650 (H)850 (D)1270 |
(W)650 (H)850 (D)1470 |
(W)650 (H)850 (D)1670 |
(W)650 (H)850 (D)1870 |
| chamber volume (litres) | 370 | 535 | 700 | 812 | 922 | 1.030 |
| external dimensions in the single-door version (mm) |
(W)1250 (H)1950 (D)1100 |
(W)1250 (H)1950 (D)1400 |
(W)1250 (H)1950 (D)1700 |
(W)1250 (H)1950 (D)1900 |
(W)1250 (H)1950 (D)2100 |
(W)1250 (H)1950 (D)2300 |
The following options are available to adapt the autoclaves exactly to your tasks:
Equipment
- House steam version, for connection to a central steam supply system
- Pass-through version with gas-tight separation for safety laboratories and clean rooms
- GMP-compliant design according to DIN 58950 and FDA regulations
- Fast water recooling via a large double jacket. In conjunction with the support pressure control via a sterile air filter, ensures the shortest possible recooling times for liquid media.
- Connection to a house supplied ring cooling system for water saving, the heat exchange takes place via a plate heat exchanger.
- Recirculation fan, further shortens the cooling time and offers the possibility of sterilisation in a steam-air mixture.
- Hot water sprinkler system (HWSS), for fast and gentle sterilization of liquids in closed vessels.
- Spray cooling, for rapid cooling of liquids in closed vessels.
- Exhaust air filter with condensate inactivation, for the sterilisation of infectious goods of risk class S2 and S3. Filter retention rate: > 99.5% 0.02µm
- Vacuum pump set with water saving device, for the safe evacuation of air from the sterile material by means of a single-stage or fractional prevacuum. Allows dry removal of the sterile material at the end of the process.
- Transport and charge trolley for ergonomic loading and unloading.
- Insert baskets and waste sterilization containers with closed bottom and lid, for optimum use of the sterilization chamber
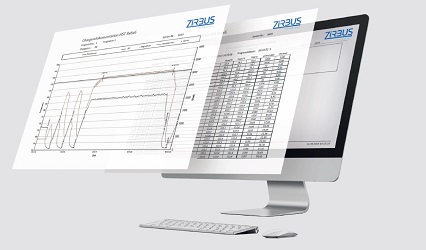
Documentation Features
- Network-compatible digital charge recording and user management system in connection with Siemens WinCC FDA 21 CFR Part 11 compliant.
- 6-channel colour line recorder for control-independent batch documentation on 120mm wide plain paper.
- 10-channel screen recorder for FDA 21 CFR Part 11 compliant digital batch documentation, network-compatible.
- USB stick for storing up to 1,000 charges on an USB stick. The data can be read out on a PC for evaluation.
- Documentation software “Sterilog” for direct documentation of the sterilization process on a PC. Communication takes place via an Ethernet interface.

- Qualification DQ, IQ and OQ according to DIN 589510 and FDS, HDS, SDS for pharmaceutical use