- Brand: Fuji Flim Wako Pure Chemical Corporation
- Product Code: PYROSTAR™ ES-F series
- Availability: Ready Stock
Manufacturer :

Endotoxin-specific LAL reagents (not activated by (1→3)-β-D-glucan), compatible with the BET (USP) compliance tests U.S Food and Drug Administration (FDA) licensed. Intended Use: Limulus amebocyte lysate (LAL) is intended for the detection of gram-negative bacterial endotoxins. PYROSTAR™ ES-F is intended for the qualitative detection of endotoxins by gel-clot or quantitative detection by kinetic turbidimetric methods.
- Endotoxin-specific lysate, avoids false positive results from glucans
- Available in multi-tests vials or single-test vials
- Can be used as either a gel-clot or Kinetic-Turbidimetric Assay (KTA) reagent
- KTA can be performed in tube reader
- Gel-Clot lysate sensitivities range from 0.015 to 0.25 EU/mL
- Available with matched control standard endotoxin (CSE)
- PYROSTAR™ ES-F reagents ( Gel-clot sensitivity 0.015 EU/mL ) are available with a KTA quantitative range of either 0.001 EU/mL to 10 EU/mL.
The KTA quantitative range is related to the Gel-Clot sensitivity. - Multi test is performed by mixing 0.1 mL of lysate reagent with 0.1 mL of sample, whereas in single test, a 0.2 mL sample directly dissolves lysate reagent.
- Multi test:
The lysate reagent which dissolve in the endotoxin test water, dispense the necessary amount in reaction tubes. - Single test:
The lysate reagent for one measurement is dispensed in advance in a test tube.
Multi Test
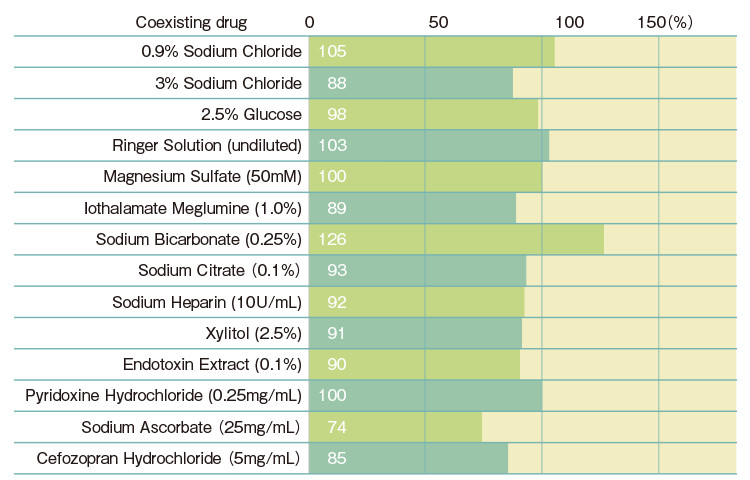
| Gel-clot Sensitivity (EU/mL) |
KTA Quantitative Range (EU/mL) |
|---|---|
| 0.015 | 0.001 to 10 |
| 0.03 to 0.25 | 0.01 to 10 |
****************************************************************************
Single Test
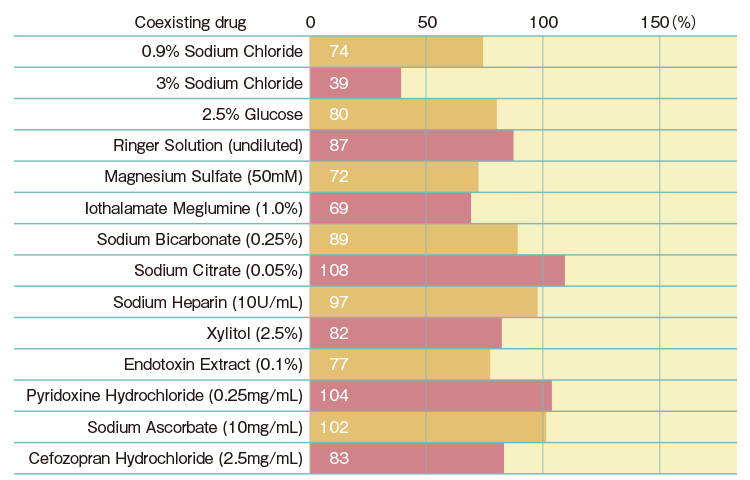
| Gel-clot Sensitivity (EU/mL) |
KTA Quantitative Range (EU/mL) |
|---|---|
| 0.015 | 0.001 to 10 |
| 0.03 | 0.01 to 10 |

